Working With Acids and Metals
For this lab we turned metals in to salt.
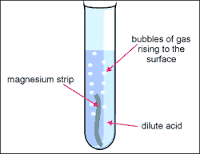
So, we started off by putting a couple strips of magnesium in acid. We left the tap a bit a open so that air could reach the tub and it wouldn’t pop. After about 4-5 minutes we took a drop of our solution and put it in backing soda to see if it was reactant or not (we know this by weather it makes bubbles or remains still). After we tested it, we took out the remaining strips of magnesium and poured the affected acid on to a glass platter. We placed it on a heater and burned it until all the water evaporated. We were left with a beautiful coat of salt (eatable). It tasted really bad, I don’t recommend it.
We also tried the same experiment with cooper. It took a lot more time however. And also, the acid in the cooper bottle heated up so much that w had to put it in iced water. And for some reason since it was blue because of the cold water, it became BROWN! strangeeee. We heated up and ended up with a PURPLE salt however, this time because our metal was cooper it was uneatable.